28 Dec How to bridge efficacy data within a biocidal product family

News
How to bridge efficacy data within a biocidal product family
A huge effort is required from applicants to perform efficacy studies covering all members in a biocidal product family. Is there an efficient approach to meet this requirement?
Under Article 19 (1) b of the biocidal products Regulation (BPR), authorisation will only be granted if a product is considered to be sufficiently effective. This in turn depends on whether the claims made for the activity of the product are in line with the efficacy demonstrated.
The registrant has to submit a set of efficacy studies to support this. It is crucial that these studies are performed under the conditions described in the label claim of the product. Many different efficacy studies are often needed to cover all conditions, strains, dilutions etc of a product. Most efficacy data come from laboratory-simulated use tests, usually in a two-step tiered approach. In some cases, more expensive field studies performed under actual conditions of use are also required. With smart design of the studies, the amount of efficacy testing data required for a biocidal product family can be minimised.
Biocidal product families and meta SPCs
Applicants can submit a group of biocidal products that have the same active substance(s), similar uses, composition (with specified variations) and levels of risk, and similar efficacy under a single entry, described as a biocidal product family (BPF).
It would be an enormous work for the applicant to provide efficacy studies for all members in each BPF. It is therefore common practice to bridge the efficacy data from a few products. Within a family, products can have different classifications, safety instructions, precautionary statements and risk management measures, relating to different risk and/or efficacy levels. To bring order, products can be categorised in ‘meta SPCs’ (summary of product characteristics). All products within a meta SPC have:
- a specified range in the compositions that is considered sufficiently similar;
- similar uses which are associated with a common set of risk management measures;
- the same hazard and precautionary statements; and
- a common set of first aid instructions, disposal, storage and shelf life.
It is therefore possible to do the assessment of the maximum risk and minimum level of efficacy at meta SPC level – instead of for the entire BPF.
How to bridge efficacy for a full product family?
The product containing the lowest concentration of the active substance(s) shall be the leading test material for the efficacy studies (ECHA’s Guidance on the BPR: Volume II Parts B+C). These studies must be performed under the most stringent conditions. The lowest efficacious dose/concentration determined, may then also be applied for the other products within that meta SPC. Furthermore, the influence of the co-formulants on the efficacy should be taken into account.
If it is difficult to single out a worst-case product, e.g. for PT 6 -22, a ‘dummy’ product may be used to cover all products in a meta SPC, containing the lowest combination of concentrations of actives and synergists/co-formulants. This product is solely used for testing purposes and is not intended to be put on the market. Alternatively, several products could be tested to cover the entire meta SPC. In some cases, efficacy studies performed for a product of one meta SPC, may also be valuable for a product in another, provided that variations in co-formulants like scents, colours, etc, have no influence on efficacy. Justification is needed for the acceptance of these forms of bridging.
How to determine a worst-case test product for disinfectant BPF (PT 1-5)?
Recently new guidance has been given for creating a harmonized understanding on how to determine a worst-case test product for efficacy assessment exclusively for disinfectant BPFs (PT1 – 5) (link). According to this guidance, a worst-case test product is defined by:
- the minimum (in use) concentration of active substance(s),
- the minimum (in use) concentration of co-formulants positively affecting efficacy,
- the maximum (in use) concentration of co-formulants negatively affecting efficacy,
- physico-chemical properties (e.g. pH value) which are the least in favourite for efficacy
However, there is usually no literature data available for determining the influence of different co-formulants on efficacy. It is therefore rather difficult to conclude which co-formulants may have a positive, negative or no effect on the efficacy of the product. This means that it is usually not possible to designate a worst-case test product before performing experimental studies. To support the choice for the representative worst-case test product, performing bridging studies using fresh products was proposed.
In general, the worst-case test product is determined based on results from bridging studies and by firm waiving arguments. It’s also wise to consent with the eCA at an early stage, preferable during the pre-submission meeting.
Testing strategy for bridging studies (PT 1-5)
To support all efficacy claims made on the product label, the suspension test bridging studies are carried out under the hardest conditions:, i.e. highest soiling claimed, lowest temperature claimed and shortest contact time that is applicable.
Only the most robust strain of each claimed target organism group has to be tested, as the whole BPF will be assessed based on the worst-case test product. For instance, a PT4 BPF dossier with a claim against bacteria and mycobacteria will need bridging tests against the most tolerant of the bacterial standard strains, which often would be a mycobacterial strain.
The effect of different co-formulants on efficacy may vary and insufficiently described in literature, therefore the effect of each co-formulant on the efficacy has to be determined by providing bridging studies. Where applicable, bridging studies may be waived, if a scientifically robust justification can be given. All waiving arguments for bridging studies, literature data or physico-chemical considerations will be evaluated by CAs and if found to be scientifically insufficient they will need to be supported by new data.
When bridging studies for PT1 – 5 are desired, experimental data may need to be generated using:
- existing products in the family containing the minimum level of active substance(s) and the co-formulant in question at the lowest and highest concentration. This approach is only valid in case the difference is clearly due to that specific co-formulant, thus where the test products is identical with respect to other co-formulants that may affect efficacy.
- dummy products containing the lowest level of active substance. The amount of all relevant co-formulants should kept at 0% or their respective minimum to design a reference product. All other considerable co-formulants should be compared to that dummy product by making another product, which is identical to the reference product with the exception of the respective co-formulant being at its maximum concentration.
It’s worth mentioning that co-formulants present across the entire BPF at a fixed nominal concentration do not need bridging/justification. The composition of the worst-case test product should have these co-formulants at their fixed concentration.
Approach for non-disinfectant BPFs (PT 6-22) by using a dummy product
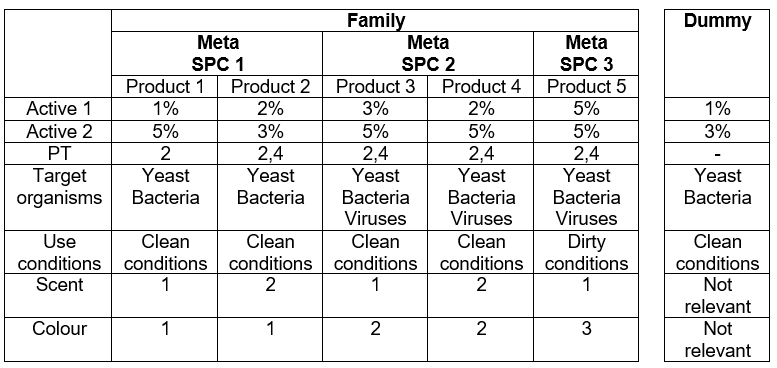
For BPFs where PT1-5 is not in scope, a different strategy may be chosen where less scrutiny applies. The grouping into Meta SPCs may be the result of the concentration range in active 1 and/or different hazard/precautionary statements. Keep in mind that different target organisms and/or different product types do not necessarily lead to separate meta SPCs. For example, a biocidal product family consists of five products, categorised provisionally under three different meta SPCs (Figure 1). In this example, there is no product that can be considered as an appropriate worst-case product for testing. Product 1 has the lowest concentration of Active 1 and Product 2 has the lowest of Active 2. Therefore, it may be useful to formulate a dummy product, covering the lowest concentrations of both active substances. This would then contain 1% of Active 1 and 3% of Active 2.
Efficacy testing for yeast and bacteria under clean conditions should be done using this dummy product. The results would not only completely cover Product 1 and 2 but can also be used for Product 3 and 4. For these two products only, the virucidal efficacy still has to be tested. Furthermore, the dummy results can also reduce the number of tests performed for Product 5. If you want to show the product is efficacious under dirty conditions, you are also required to do so under clean conditions. The dummy results can support this.
Regarding the virucidal claim for Product 3 and 4, again a worst-case testing approach is applicable. No dummy is necessary as Product 4 is the ‘worst-case’ product. Thus, efficacy testing for viruses under clean conditions can be done only for Product 4. Again, these results may also be useful to support the claim for Product 5, as described above.This leaves the efficacy testing under dirty conditions for yeast, bacteria and viruses for Product 5, using the product itself.
A well-grounded justification is necessary for the use of bridging test results. Furthermore, it is important that variations in co-formulants, scents, colours, etc, have no influence on the efficacy. A justification is also required for this aspect to allow bridging. Also, if the dummy product is not effective enough against the target organisms, the strategy should be adapted to include the actual products. In essence, great care must be taken when determining the most suitable approach. The example deals with the most important, but does not cover all, aspects of worst-case testing. For further guidance, see ECHA’s Guidance on the BPR: Volume II Parts B+C, and specifically Chapter 5.2.2.
Figure 1. Illustration of a hypothetical non-disinfectant BPF with the individual members and their desired claims